High Purity USP EP 183133-96-2 Cabazitaxel for Anti-cancer Treatment
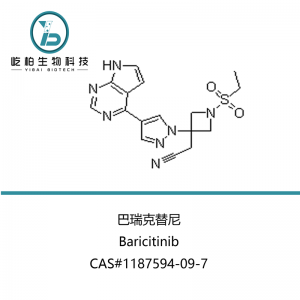
| Product name | Cabazitaxel |
| Synonyms | (αR,βS)-β-[[(1,1-DiMethylethoxy)carbonyl]aMino]-α-hydroxybenzenepropanoic Acid (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-(Acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-11-hydroxy-4,6-diMethoxy-4a,8,13,13-tetraMethyl-5-oxo-7,11-Methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl Ester |
| CAS No. | 183133-96-2 |
| Appearance | White to almost white crystalline solid |
| Molecular Formula | C45H57NO14 |
| Molecular Weight | 835.93 |
| Application | Pharma grade or research purpose |
| Packing | As per your request |
| Storage | Preserve in tight,light-resistant containers in a cool place |
|
Cabazitaxel CAS:183133-96-2 |
||
| ITEMS | STANDARDS | RESULTS |
| CHARACTERISTICS | White to almost white crystalline solid | White crystalline solid |
| IDENTIFICATION | Should to positive | Positive |
| SOUBILITY | Freely soluble in Acetone and Dichloromethane,Soluble in Ethanol and Methanol, Sparingly soluble in Ethyl acetate. | Complies |
| LOSS ON DRYING | ≤1.0% | 0.1% |
| RESIDUE ON IGNITION | ≤0.2% | 0.05% |
| HEAVY METALS | ≤20ppm | Complies |
| RELATIVE SUBSTANCE | Single impurity:≤0.3% | 0.08% |
| Total Impurities:≤0.8 % | 0.19% | |
| RESIDUAL SOLVENT | Tetrahydrofuran:≤0.072%Methanol: ≤0.3%
Dichloromethane:≤0.06% Ethyl Acetate: ≤0.5% |
Not DetectedNot Detected
Not Detected Not Detected |
| ASSAY | NLT 98.5% | 99.78% |
| Conclusion:It complies to prescribed enterprise standard | ||
Company Information
√ Management layer’s full experience in factory and skilled technicians followers; √ Quality is always our top consideration, Strict QC system; √ 11 years experienced exporting sales team; √ Independent R&D lab; √ Two signed long term GMP workshops; √ Rich resources of plenty idle factories for customized project; √ High Efficiency working team with consistent path.


Write your message here and send it to us