Pharmaceutical Grade 1392275-56-7 Tenofovir alafenamide fumarate For Treatment of Chronic Hepatitis B
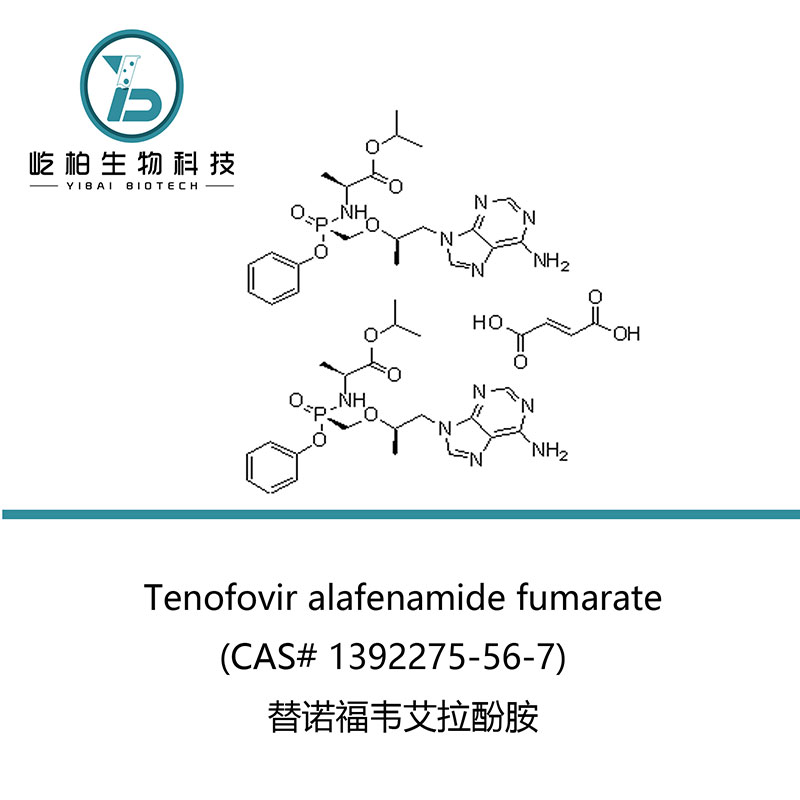
| Product name | Tenofovir alafenamide fumarate |
| Synonyms | N-[(S)-[[(1R)-2-(6-Amino-9H-purin-9-yl)-1-methylethoxy]methyl]phenoxyphosphinyl]-L-alanine 1-methylethyl ester (2E)-2-butenedioate (2:1); GS 7340-03 |
| CAS No. | 1392275-56-7 |
| Appearance | White or almost white crystalline powder |
| Molecular Formula | 2(C21H29N6O5P).C4H4O4 |
| Molecular Weight | 1069.02 |
| Usage | Pharmaceutical Grade for Research purpose |
| Packing | As per your request |
| Storage | Preserve in tight,light-resistant containers in a cool place |
Company Information
√ Management layer’s full experience in factory and skilled technicians followers; √ Quality is always our top consideration, Strict QC system; √ 11 years experienced exporting sales team; √ Independent R&D lab; √ Two signed long term GMP workshops; √ Rich resources of plenty idle factories for customized project; √ High Efficiency working team with consistent path.


Write your message here and send it to us